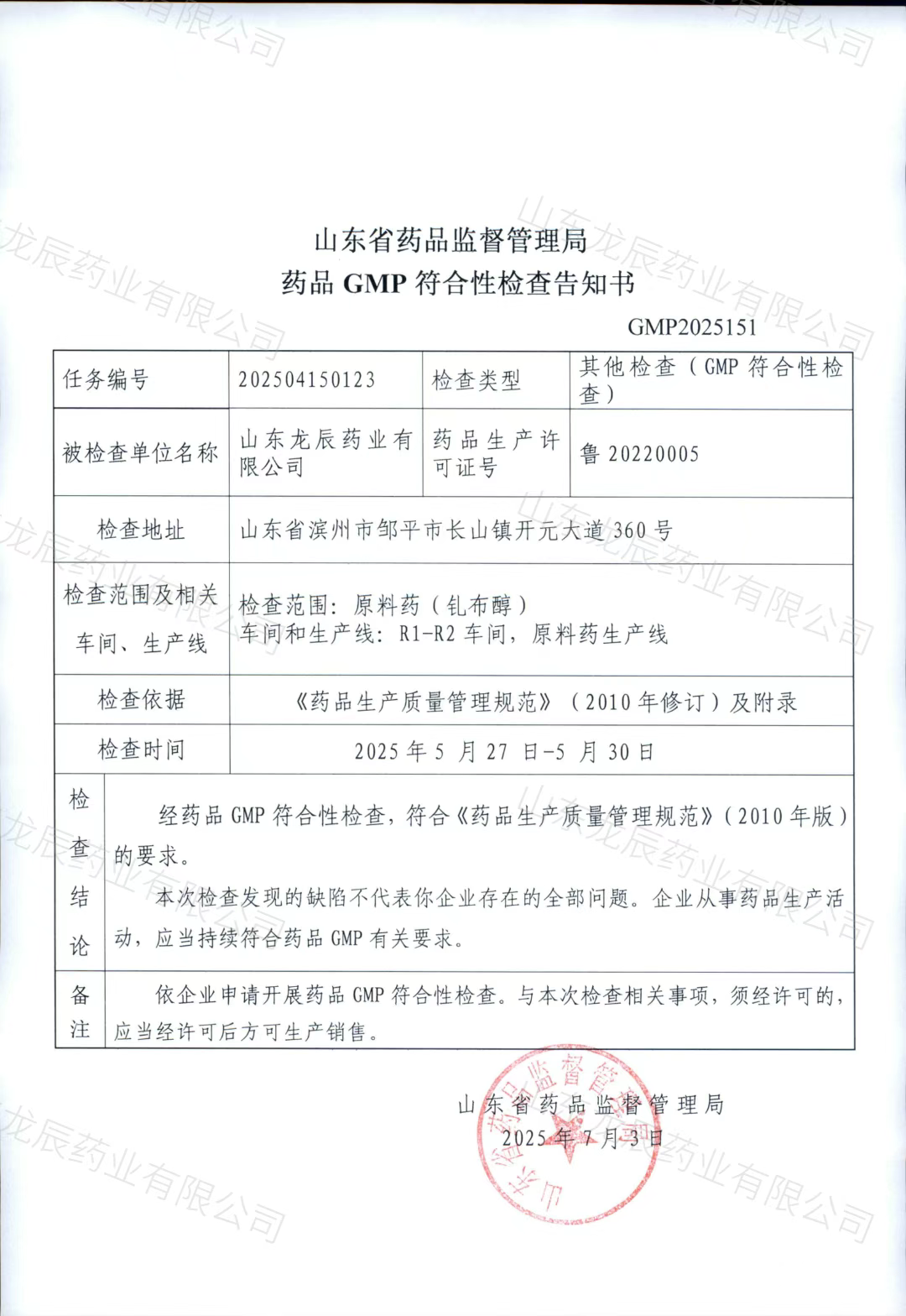
The issuance of this notification marks the launch of full-scale production and commercial distribution of Gadobutrol, and represents a significant breakthrough in Longzen’s core business development. With this achievement, Longzen enters a new era of accelerated growth in the pharmaceutical industry.